The R&D department plays a central role within the Company for both the activity to develop new products and the constant improvement of current production process.
Our R &D department is constant relation with the academic sector. This relationship provides us access to specialists in areas such as Immunology, Molecular Biology, Microbiology and Biotechnology, essential to successfully implement R&D developments and projects.
Conducting clinical trials to evaluate both safety and efficacy of vaccines, it is of critical importance for the R & D process. For this purpose, we perform trials in experimental fields with different animal species, following the highest international standards.
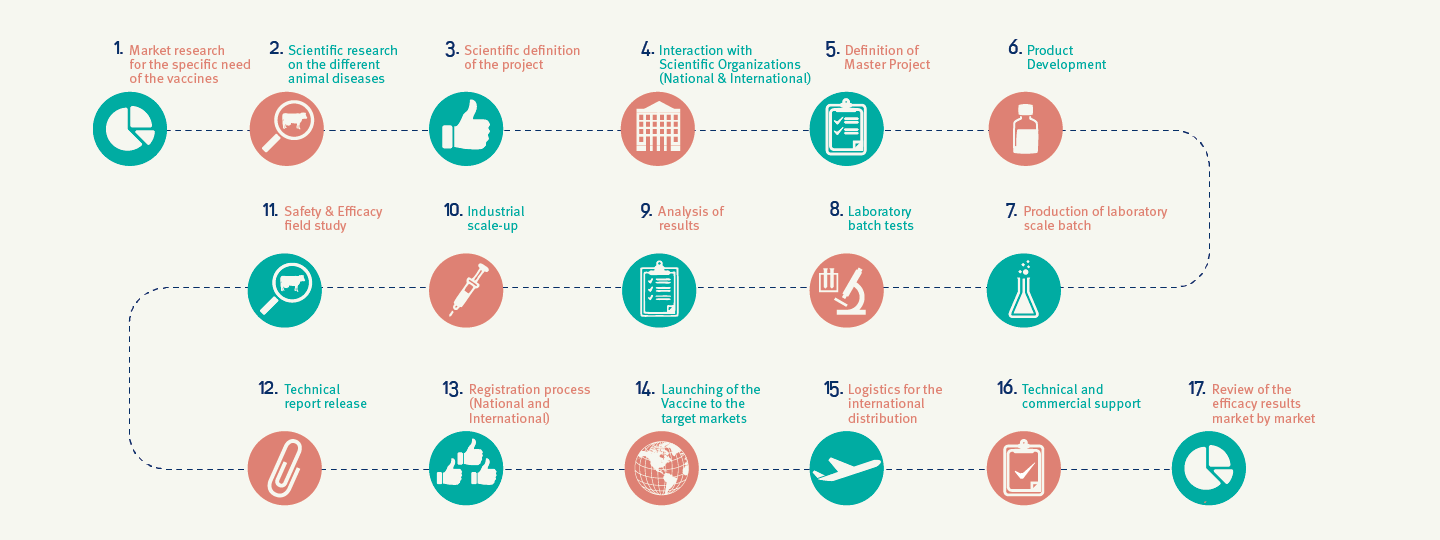
Concentrating 100% of technical efforts on developing and producing only biological products, allows us to increase our knowledge and as a result obtain a highly specialized product.
Our vaccines have proven highly effective in each of the markets in which we participate, and the results obtained at the field level support the quality of our products.

INTERNATIONAL STANDARS:
We work under the most relevant and demanding International Standards in biological production (US CFR, PhEur, OIE, WHO, and Mercosur GMP). Our production facilities, located in Montevideo – Uruguay, are built under strict biosecurity and environmental care conditions.
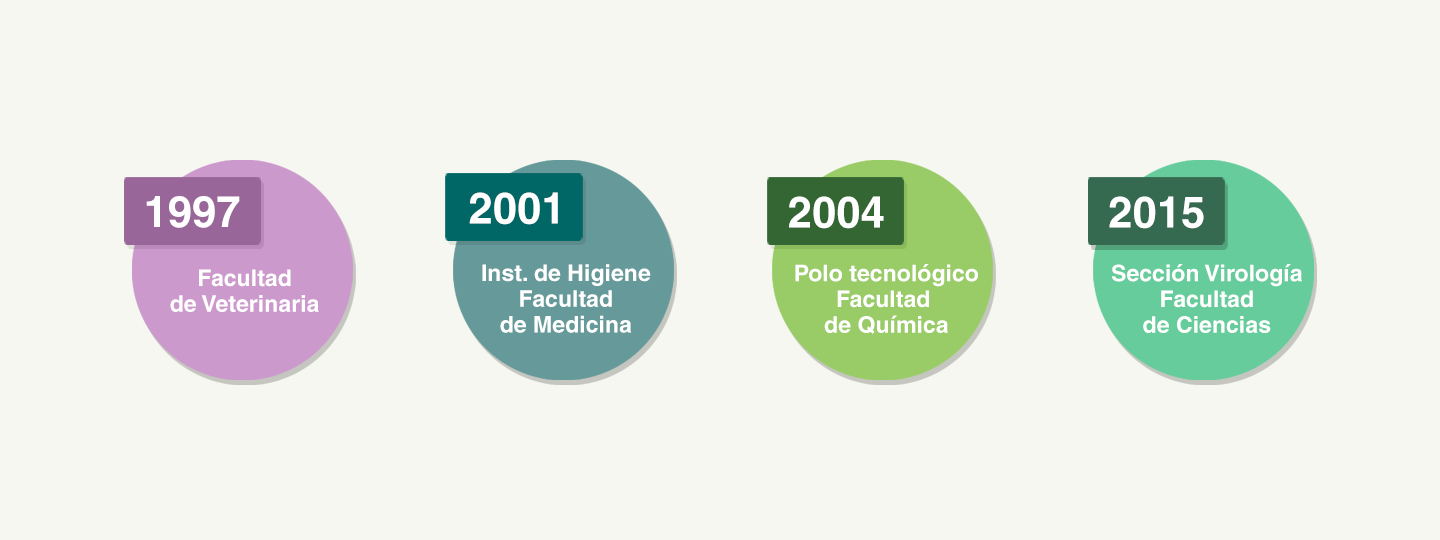
In order to strengthen our research and development activities, from the beginning we have formed alliances with different actors in the academic sector. Several development projects, both for new products and for the improvement of existing processes, have been carried out jointly with groups of researchers from Faculty of Medicine, Faculty of Chemistry, Faculty of Veterinary Science, Faculty of Sciences, ORT University, among others.

